Ever get that sharp, slightly minty, but mostly chemical smell in a lab and wonder exactly which solvent is hitting your sinuses? If you’re hanging around organic synthesis or industrial coating facilities, there’s a solid chance you’re smelling 2-methylpentan-3-one. Most people just call it ethyl isopropyl ketone. It’s one of those unsung workhorses of the chemical world that doesn’t get the "celebrity" status of acetone or benzene, but honestly, we'd be in a bit of a spot without it.
It's a branched ketone.
That branch—that little methyl group hanging off the second carbon—changes everything. It’s not just a longer version of MEK (methyl ethyl ketone). It has its own personality, its own boiling point quirks, and a specific way it interacts with polymers that makes it indispensable for certain high-end applications.
The Anatomy of 2-methylpentan-3-one
Let's look at the skeleton. You have a five-carbon chain, but it’s not a straight line like pentan-3-one (diethyl ketone). Instead, you've got a carbonyl group sitting pretty on the third carbon, and a methyl group branching off the second. This structural nuance is why chemists use the IUPAC name 2-methylpentan-3-one, though in a fast-moving industrial setting, "ethyl isopropyl ketone" is what you'll hear shouted over the hum of the machinery.
The formula is $C_6H_{12}O$. It’s an isomer of hexanone. But don't mistake it for methyl isobutyl ketone (MIBK), which is a much more common industrial solvent. They're like cousins—similar hobbies, but very different temperaments when it comes to solubility parameters and evaporation rates.
2-methylpentan-3-one is a clear, colorless liquid. It flows easily. If you spill it (don't), you'll notice it evaporates slower than acetone but faster than some of the heavier glycol ethers. This "middle-of-the-road" volatility is exactly why it’s sought after.
Why the Molecular Shape Actually Matters
If you're wondering why we care about that methyl branch, think about how molecules pack together. In a straight-chain ketone, the molecules can get relatively close. But that isopropyl group on one side of the carbonyl creates "steric hindrance." It’s bulky. It’s awkward.
This bulkiness affects how it sits in a solution. When you’re trying to dissolve a specific resin—say, for a high-performance automotive coating—you need a solvent that can wiggle into the polymer chains and push them apart. 2-methylpentan-3-one is particularly good at this for certain vinyl and acrylic resins. It provides a level of "bite" that simpler ketones sometimes lack.
Actually, it’s also a key intermediate.
When people talk about the "fine chemicals" industry, they’re often talking about using molecules like this as building blocks. You don't just use it to dissolve stuff; you use it to make stuff. Through a process called reductive amination, you can turn this ketone into various amines which eventually end up in pharmaceuticals or agricultural chemicals. It’s a precursor. A starting point.
💡 You might also like: The Amazon Fire Phone: What Really Happened to Jeff Bezos's 3D Dream
Industrial Reality vs. Lab Theory
In a textbook, 2-methylpentan-3-one is a clean line drawing. In a 55-gallon drum in a warehouse, it’s a commodity. It’s usually produced by the catalytic hydrogenation of mesityl oxide or through the aldol condensation of smaller ketones followed by hydrogenation.
If you look at the safety data sheets (SDS) from major suppliers like Shell or ExxonMobil (though they often focus on the more common MIBK), you'll see the standard warnings. It’s flammable. The flash point is around 15°C to 20°C. That’s basically "room temperature" on a warm day, meaning it can form explosive mixtures with air quite easily.
I’ve talked to technicians who prefer it over MIBK for specific cleaning cycles because it leaves less residue in certain vacuum systems. Is that a universal truth? Maybe not. But in the niche world of precision parts cleaning, these small differences in boiling points ($113°C$ to $115°C$ for 2-methylpentan-3-one) matter immensely.
Safety, Health, and the "Hidden" Risks
We need to be real about the health side. Like most ketones, breathing it in isn't going to do your nervous system any favors. It’s a CNS depressant if you huff enough of it. You’ll get dizzy, maybe a headache, and eventually, if you're really exposed, you're looking at more serious narcosis.
But there’s a nuance here.
Ketones are often criticized for their "synergistic" toxicity. This means that while 2-methylpentan-3-one might not be super toxic on its own, if it’s mixed with something like n-hexane, it can actually speed up the way your body processes the other chemical, making the overall mixture more dangerous. This is something often overlooked in small-scale shops that mix their own solvent blends.
- Skin contact: It strips the oils right off your hands. Do it enough, and you get dermatitis. Use nitrile gloves, not latex. Latex will swell up and fail faster than you’d think.
- Inhalation: Use a fume hood. If you can smell it strongly, you're likely above the recommended exposure limits.
- Environmental: It’s not particularly "persistent" like a PFAS chemical, but it’s still an organic pollutant. It’s got a high VOC (Volatile Organic Compound) count, which means regulators are always keeping an eye on how much of this stuff is being vented into the atmosphere.
2-methylpentan-3-one in Synthetic Chemistry
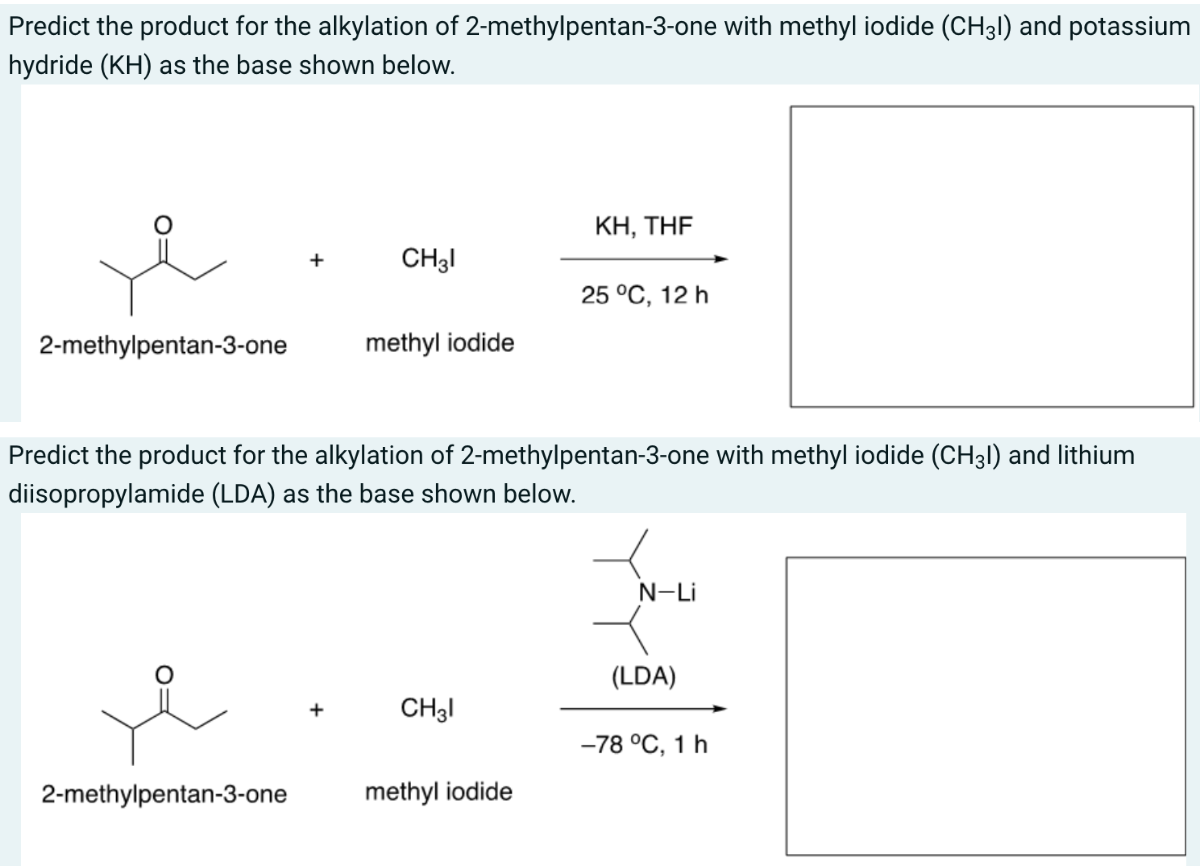
For the organic chemistry nerds out there, this ketone is a playground. Because it’s unsymmetrical, you have two different sites where you can form an enolate. However, the isopropyl side is much more crowded.
If you try to do a base-catalyzed reaction, the base is almost always going to grab a proton from the ethyl side (the "less hindered" side). This regioselectivity is a gift for synthetic chemists. It allows them to predict exactly where a new bond will form. If you were using a perfectly symmetrical ketone like 3-pentanone, you wouldn't have that control; the molecule could react at either side and you’d end up with a messy mixture of products.
Common Misconceptions and Comparisons
I often see people confuse this with 3-methyl-2-pentanone. It’s an easy mistake. The numbers are just swapped, right?
Wrong.
The position of the "C=O" (carbonyl) group changes the entire boiling point, the polarity, and how it reacts with your nose. 3-methyl-2-pentanone is a "methyl ketone," which makes it much more reactive in certain tests (like the iodoform test). 2-methylpentan-3-one is a "dialkyl ketone" where both sides of the carbonyl are carbon chains. It’s more stable, less reactive to oxidation, and generally behaves more "stubbornly" in the lab.
📖 Related: The Gilded 6 Bits: Why This Early Computing Quirk Still Matters Today
Actionable Insights for Handling and Sourcing
If you're actually looking to work with this stuff, don't just buy the first "reagent grade" bottle you see.
- Check the Purity: For solvent use, 98% is usually fine. But if you’re using it as a precursor for a pharmaceutical intermediate, you need to watch out for "isomeric impurities." Sometimes, small amounts of other $C_6$ ketones stay in the mix.
- Storage Matters: Store it under nitrogen if you want it to last. While it's more stable than aldehydes, ketones can still slowly form peroxides or undergo self-condensation over years if exposed to light and oxygen.
- Waste Disposal: Don't pour it down the sink. Seriously. It’s a fire hazard in the plumbing and it’s illegal. Collect it in a dedicated "non-halogenated organic waste" container.
- Substitution: If you’re trying to find a "greener" alternative, look at ethyl lactate or certain terpene-based solvents. They won't always work—especially if you need that specific ethyl-isopropyl branching—but they’re better for the planet.
2-methylpentan-3-one is a specialized tool. It’s the "offset screwdriver" of the chemical world. You don’t need it for every job, but when you’re stuck in a corner with a specific resin solubility issue or a regioselective synthesis problem, nothing else quite fits the bill. Understand the structure, respect the flash point, and it’ll be one of the most reliable molecules in your inventory.
Next Steps for Implementation:
- Verify the compatibility of your specific polymer resin using the Hansen Solubility Parameters for 2-methylpentan-3-one (look for $\delta_d$, $\delta_p$, and $\delta_h$ values).
- Conduct a small-scale "evaporation rate" test against your current solvent to see if the transition to a branched ketone improves surface leveling in your coatings.
- Audit your PPE—ensure your respirators are equipped with Organic Vapor (OV) cartridges, as standard dust masks offer zero protection against ketone vapors.