You’re probably here because you’re staring at a bucket of cleaning supplies or smelling something funky near a pool and wondering if you’re about to see a "death cloud." Most people searching for a picture of chloramine gas expect to find a vivid image of a thick, green-yellow mist rolling across a kitchen floor.
Honestly? It doesn't usually look like that.
Chloramine gas is a sneaky, colorless-to-pale-yellow chemical compound that forms when you make the classic, dangerous mistake of mixing bleach with ammonia-based cleaners. It’s not like the movies. There’s no dramatic, neon-colored vapor most of the time. In fact, by the time you can actually "see" a cloud, you’re already in significant trouble. Because the gas is often invisible to the naked eye at low but toxic concentrations, people stay in the room way too long. They keep scrubbing. They keep breathing it in. That's how a Saturday morning chore turns into a trip to the ER.
What Does Chloramine Actually Look Like?
If you were to look at a high-resolution picture of chloramine gas captured in a controlled laboratory setting, you might see a very faint, yellowish tint if the concentration is high enough. But in a real-world setting—like your bathroom—it basically looks like clear air.
Don't let the lack of a "scary cloud" fool you.
🔗 Read more: Zinc supplements: What most people get wrong about your immune system
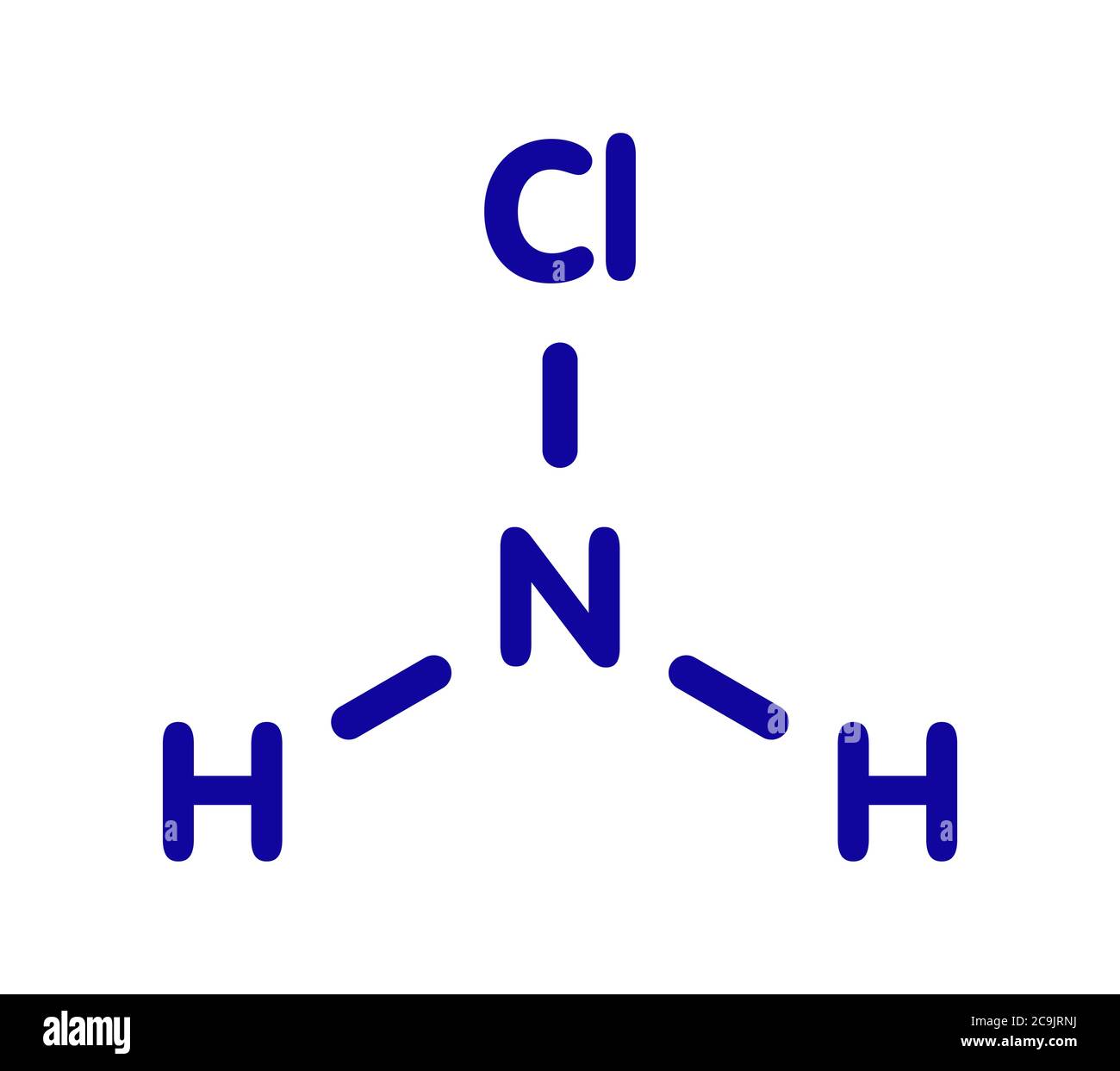
Chemically, we’re talking about monochloramine ($NH_{2}Cl$), dichloramine ($NHCl_{2}$), and nitrogen trichloride ($NCl_{3}$). When you mix sodium hypochlorite (bleach) with ammonia, a series of rapid reactions occur. First, you get monochloramine. If there’s more bleach present, it reacts further to create dichloramine and then nitrogen trichloride.
The "pool smell" everyone knows? That’s not actually chlorine. It’s chloramines. When the chlorine in a pool reacts with nitrogen-rich organic matter—basically sweat, oils, and, yes, urine—it creates these gases. So, if you want a mental picture of chloramine gas, don't think of a laboratory beaker; think of that stinging, sharp "gym pool" odor that makes your eyes water. That smell is the gas physically hitting your mucous membranes.
The Chemistry of the Disaster
It happens fast. You’ve got a stubborn stain. You pour some bleach. It’s not working, so you grab the "blue stuff" or a floor cleaner that happens to have ammonium hydroxide in it.
The reaction looks like this:
$NH_{3} + NaOCl \rightarrow NH_{2}Cl + NaOH$
That’s the birth of monochloramine.
It’s an exothermic reaction, meaning it can sometimes produce heat. If you’ve ever seen a "bubbling" or "fizzing" in your sink after mixing chemicals, that’s the physical manifestation of the gas being released. Even if the air looks clear, those bubbles are your warning sign. Experts like those at the National Capital Poison Center (Poison Control) deal with thousands of these calls annually. They’ll tell you that the lack of a visible "picture" is exactly why it's so hazardous. You don't see the border of the danger zone.
Why People Get Confused with Chlorine Gas
There is a huge overlap in Google searches between chloramine and chlorine gas. They aren't the same.
Chlorine gas ($Cl_{2}$) is what happens when you mix bleach with an acid (like vinegar or some toilet bowl cleaners). Chlorine gas is more likely to show up as a visible, greenish-yellow cloud. It’s heavier than air and stays low to the ground.
Chloramine, however, is the result of the bleach-ammonia crossover. While both are respiratory irritants, chloramine is particularly nasty because it’s so common in household accidents. People think they’re being "extra clean." They aren't. They’re just creating a chemical weapon in a small, unventilated space.
Real-World Scenarios Where You’ll Encounter It
- The "Super Clean" Bathroom: This is the classic. Mixing window cleaner (ammonia) with bleach. You won't see a cloud. You'll just suddenly feel like you can't take a full breath. Your throat will feel "tight."
- Industrial Spills: In large scale settings, like water treatment plants, a leak might produce a visible haze. In these cases, a picture of chloramine gas would look like a shimmering distortion in the air, similar to heat waves on a hot road.
- Indoor Water Parks: If the ventilation is poor, the concentration of nitrogen trichloride (the most volatile chloramine) builds up just above the water's surface. It’s why competitive swimmers often have chronic coughs, sometimes called "pool lungs."
The Symptoms: Knowing When to Run
Since you can’t rely on a picture of chloramine gas to alert you, you have to rely on your body.
It starts with the eyes. They’ll sting and turn red. Then comes the nose—a burning sensation that feels like you’ve inhaled "spicy" air. If you stay in the room, the gas reacts with the moisture in your lungs. This is the scary part. It forms small amounts of hydrochloric acid and free radicals upon contact with your wet tissues.
You’ll start coughing. It's a dry, hacking cough. Your chest might feel heavy. For people with asthma or COPD, this can be fatal. Even for healthy people, high exposure can lead to pulmonary edema (fluid in the lungs) or pneumonitis.
What to Do Instead of Taking Photos
If you see a fizzing mixture or smell that sharp, acrid scent: Leave. Now.
Don't stop to pour it down the drain unless you can do it in one second while holding your breath. Don't try to "neutralize" it with another chemical—that usually makes it worse.
- Evacuate: Get to fresh air immediately.
- Ventilate: If you can safely reach a window on your way out, open it. Turn on the exhaust fan.
- Call for Help: If you’re coughing uncontrollably or your chest hurts, call 911. Otherwise, call Poison Control at 1-800-222-1222.
- Dilute: Once the "cloud" (visible or not) has dissipated—usually after several hours of heavy ventilation—the remaining liquid should be diluted with massive amounts of water while wearing protective gear.
The Myth of the "Green Mist"
We need to debunk the "green mist" trope.
Most images online labeled as a picture of chloramine gas are actually stock photos of smoke bombs or digital renders. In reality, a toxic concentration of chloramine can exist in a room that looks perfectly normal. This is the "silent" nature of household chemical accidents. If you are waiting to see a color change before you exit the room, you have waited too long.
The concentration matters. In professional water treatment, chloramine is actually used as a disinfectant because it’s more stable than chlorine. It stays in the pipes longer. When you turn on your tap, there are tiny, safe amounts of chloramines in that water. You’re drinking it. It’s clear. This proves that the substance itself doesn't have an inherent, bold color at functional levels.
Actionable Safety Steps
First, go to your cleaning cabinet. Read the labels. If a bottle says "contains bleach" or "sodium hypochlorite," move it to a different shelf than anything containing "ammonia" or "ammonium hydroxide."
Physical separation is the only way to prevent a "tired brain" mistake at 11:00 PM when you’re trying to scrub a floor.
Second, never mix cleaners. Period. Even "natural" cleaners like vinegar can react poorly with bleach.
✨ Don't miss: Learn to Jump Rope: Why You’re Failing and How to Actually Get Good
If you suspect you've been exposed, don't just "tough it out." The inflammation in the lungs can sometimes peak hours after the initial exposure. If you find yourself wheezing three hours later, go to an urgent care.
Final Reality Check
Finding a genuine picture of chloramine gas is difficult because the gas is mostly transparent. The danger isn't in what it looks like; it's in what it does to your respiratory system.
Stop looking for a visual cue. Trust your nose and your lungs. If the air smells "sharp" or "chemical," and you’ve been mixing products, the science says you’re in danger. Walk away, get to fresh air, and let the room vent out for a full day before you even think about going back in to finish the job. Your health is worth more than a clean bathtub.
Immediate Next Steps:
- Check your "Blue" glass cleaners for ammonia content.
- Check your laundry bleach for clear warning labels.
- Store these chemicals in separate rooms or cabinets to eliminate the risk of accidental mixing.
- Save the Poison Control number in your phone now: 1-800-222-1222.