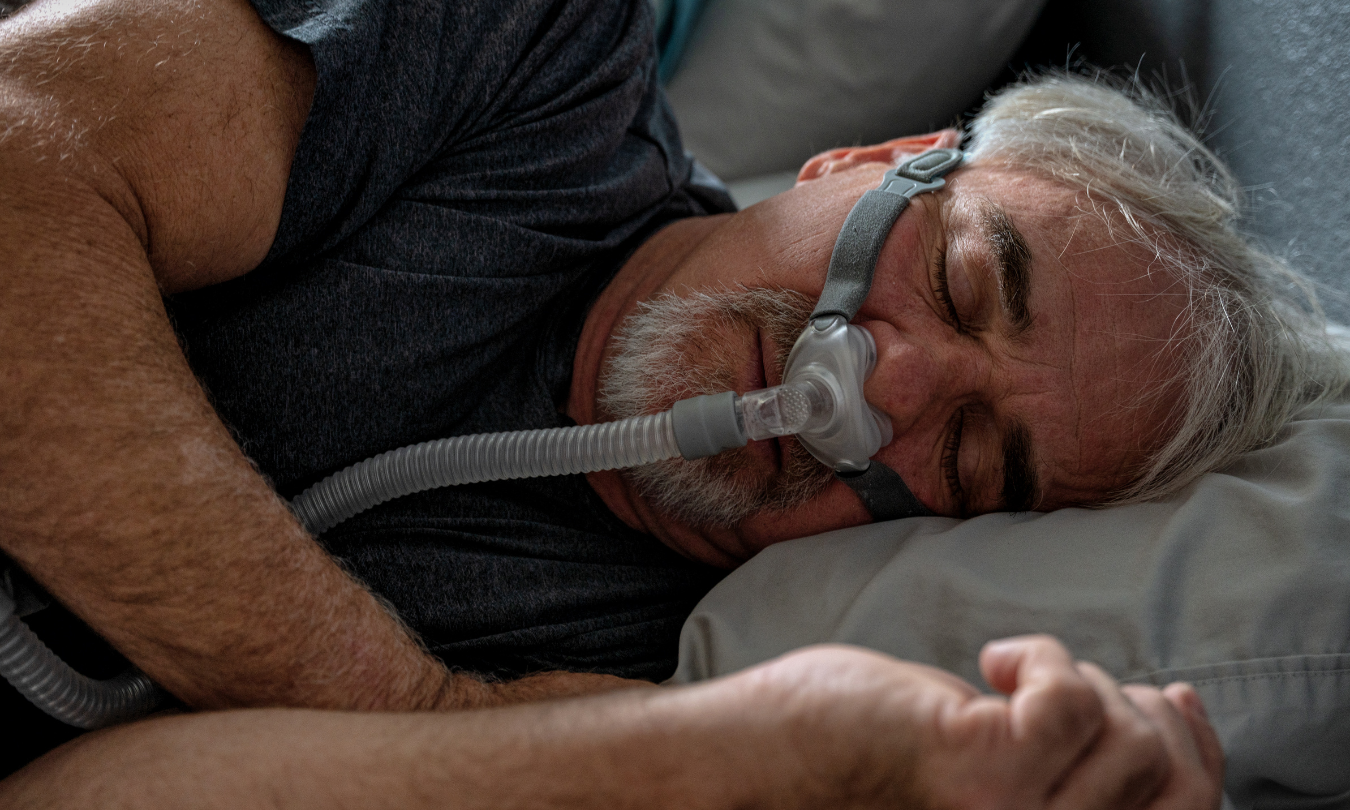
It started with black specks. People using DreamStation machines began noticing tiny, dark debris in their water chambers and masks. They didn't know it then, but those flakes were the first signs of a massive safety crisis. In June 2021, Philips Respironics issued a voluntary recall for millions of sleep apnea and ventilator devices. The problem? A specific type of foam used for soundproofing—polyester-based polyurethane (PE-PUR) foam—was breaking down. When it degraded, it could be inhaled or swallowed by the person trying to breathe. This wasn't just a minor technical glitch. It was a health emergency that left millions of patients stuck between a rock and a hard place: use a potentially toxic machine or stop treating their sleep apnea and risk heart failure or stroke.
Honestly, the fallout has been messy.
Even years later, the Philips CPAP machine recall remains a primary concern for patients and doctors alike. We're talking about roughly 15 million devices globally. The FDA eventually gave this a Class I recall status, which is the most serious kind. It means using the product could cause "serious adverse health consequences or death." That’s a heavy weight for someone who just wants a good night's sleep.
The Real Danger Inside the Foam
The science of why this happened is actually pretty straightforward, but the consequences are complex. Philips used the PE-PUR foam to make the machines quieter. They wanted a premium, silent experience. However, they found that in high-heat and high-humidity environments, that foam starts to crumble.
It gets worse if you use ozone cleaners. Many people bought expensive SoClean units or similar products thinking they were doing the right thing by "sanitizing" their gear without water. In reality, the ozone was accelerating the chemical breakdown of the foam. When the foam degrades, it releases "volatile organic compounds" (VOCs).
Think about that for a second.
You’re asleep. Your airway is being kept open by a pressurized stream of air. But that air is passing over a foam filter that is literally disintegrating into microscopic particles and gas. You’re breathing in chemicals like toluene diamine and diisocyanate. According to the FDA, the risks of inhaling these include inflammation, respiratory issues, and potential "toxic and carcinogenic effects."
Why the Replacement Process Was a Headache
If you were one of the millions affected, you likely felt ignored for a long time. Philips launched a massive repair and replacement program, but it didn't exactly go smoothly. They initially tried to replace the bad foam with a new silicone-based foam.
Then came another hurdle.
The FDA grew concerned about the new silicone foam too. After an inspection of a Philips facility in Pennsylvania, they found that the new material also failed some safety tests in a different way. While it didn't have the same "crumbling" issue as the PE-PUR, there were questions about its stability and potential off-gassing. This created a massive backlog. Patients were told to register their serial numbers on a portal and wait. And wait. Some waited over two years for a refurbished machine.
A Breakdown of the Affected Devices
It wasn't just the popular DreamStation. The list was huge.
👉 See also: Articles on family violence: Why the headlines often miss the real story
- DreamStation CPAP, Auto CPAP, and BiPAP (The most common ones)
- DreamStation GO (The travel version)
- SystemOne (Q-Series)
- RemStar SE Auto
- Trilogy 100 and 200 ventilators
- A-Series BiPAP Hybrid A30 and A40
If you have a machine sitting in a closet and you aren't sure, look at the bottom. There's a serial number. You can still check it on the Philips recall website, though the landscape has shifted toward legal settlements now.
The 2024 Consent Decree and What It Changed
In early 2024, the situation reached a breaking point. The U.S. Department of Justice, acting on behalf of the FDA, stepped in with a "Consent Decree of Permanent Injunction." This is basically the government putting Philips in a corner.
Under this decree, Philips Respironics is essentially banned from selling new CPAP or BiPAP machines in the United States until they meet very specific, very rigorous safety requirements. They can still service existing machines and provide parts, but their days of dominating the market with new DreamStation units are paused indefinitely. This was a massive win for consumer safety, but a huge blow to the company's business.
It also means that if you’re looking for a new machine today, your doctor is almost certainly prescribing a ResMed or a Fisher & Paykel device. Philips is out of the game for the foreseeable future in the U.S. market.
The Massive $1.1 Billion Settlement
Money doesn't fix lungs, but it does provide some accountability. In April 2024, Philips agreed to pay $1.1 billion to settle personal injury and medical monitoring claims in the U.S. This was a huge moment.
Before this, there was a separate $479 million settlement just for the economic loss—meaning, the money people spent on the machines themselves. The $1.1 billion is different. That is for people who claim they actually got sick—developed lung cancer, asthma, or chronic COPD—because of the Philips CPAP machine recall issues.
The company hasn't admitted fault in these settlements, which is standard legal maneuvering, but the sheer size of the payout speaks volumes. They wanted to cap their liability because the number of lawsuits was growing exponentially.
What Most People Get Wrong About the Recall
A lot of folks think that if their machine looks fine, it is fine. That's a mistake. The degradation often happens on a microscopic level before you ever see a black speck in the mask. By the time you see debris, you've likely been inhaling VOCs for months.
Another misconception? That you should just stop using your CPAP immediately.
Doctors actually advise against this for many patients. If you have severe obstructive sleep apnea (OSA), the risk of dying from a heart attack tonight because you stopped your therapy might actually be higher than the long-term risk of the foam. It's a terrifying choice. The official advice has always been: talk to your pulmonologist. Don't just pull the plug. They might switch you to a different brand or, in some cases, tell you to keep using the Philips machine with an inline bacterial filter while you wait for a replacement. (Though it's worth noting, the FDA said filters don't stop the gas/VOCs, only the particles).
Looking Ahead: Is the New Foam Safe?
This is the million-dollar question. Philips replaced the old PE-PUR foam with a silicone-based material. They claim it’s safe. However, the FDA’s 2021 inspection report raised red flags about the new silicone foam potentially releasing chemicals too.
Recent data from Philips suggests that the new machines are performing well in testing, but many patients have lost trust. It's hard to go back to a brand after you've been told your life-saving device might have been poisoning you.
If you are currently using a "remediated" DreamStation (one that was sent back to you with new foam), keep a close eye on it. If you smell anything "chemical-like" or "sweet" coming from the air, stop using it and call your doctor. That's often a sign of off-gassing.
How to Protect Yourself Now
If you are still dealing with the aftermath of the Philips CPAP machine recall, you have to be your own advocate. Don't wait for a letter in the mail that might never come.
First, confirm your device's status. Go to the Philips recall portal and enter your serial number. If it says your machine is affected and you haven't received a replacement, call them. Be persistent.
Second, check your health. If you’ve used an affected machine for years and have developed new respiratory issues, coughs, or sinus problems, document them. Mention the recall to your primary care doctor so it’s in your medical records. This is crucial if you ever decide to join the medical monitoring part of the legal settlements.
👉 See also: Why You Should Talk to Someone Before the Pressure Breaks You
Third, look at your cleaning habits. If you are using a new machine—regardless of the brand—stop using ozone cleaners. Most manufacturers, including ResMed, have stated that ozone can damage the machine and void your warranty. Stick to mild soap and water. It's boring, but it's safe.
Finally, explore your options for a brand switch. Many insurance companies have created "fast-track" approvals for patients to move away from Philips devices. Talk to your DME (Durable Medical Equipment) provider. Tell them you no longer feel comfortable with the brand. You might be eligible for a ResMed AirSense 10 or 11 sooner than you think.
Immediate Action Steps for Impacted Patients
- Verify the Serial Number: Locate the sticker on the bottom of your device and enter the code into the Philips Respironics recall website to see if your specific unit is part of the 2021-2024 actions.
- Consult Your Specialist: Schedule a brief appointment with your sleep doctor to discuss the "benefit vs. risk" ratio. Do not make the decision to stop therapy unilaterally.
- Register for Settlements: If you haven't already, look into the "Economic Loss Settlement" to see if you are owed a refund for the device itself. If you have health issues, contact a legal professional regarding the personal injury settlement.
- Inspect the Air Path: Even if you don't see black specks, check the "outflow" port of your machine for any discoloration or sticky residue.
- Switch Cleaning Methods: Immediately cease the use of any UV or Ozone-based cleaning systems. Use simple distilled water and fragrance-free dish soap for your hose and mask.
- Request a Brand Change: Contact your insurance provider and state that you are requesting a "brand-specific" replacement due to the Class I recall. Many providers are now prioritizing these requests to mitigate their own liability.
The era of Philips dominating the CPAP market has changed forever. While the company works through its legal and regulatory hurdles, the priority remains the millions of people who just want to breathe safely while they sleep. Stay informed, keep your records organized, and don't settle for a machine that makes you feel unsafe.