If you drop a human red blood cell into a glass of pure, distilled water, it won’t survive more than a few seconds. It basically explodes. It's violent, microscopic, and entirely predictable if you understand the laws of physics.
We call this process hemolysis.
👉 See also: In a Different Key: What Everyone Gets Wrong About the History of Autism
Most people remember the term "osmosis" from high school biology, but they usually forget how it actually works in a life-or-death situation for your blood. Your body spends an incredible amount of energy making sure your blood plasma stays salty enough to keep your cells from popping. If you ever find yourself hooked up to an IV, the fluid going into your veins isn't just water; it’s a saline solution designed to match the salt concentration of your cells.
If a doctor accidentally used pure water instead of saline, the results would be catastrophic.
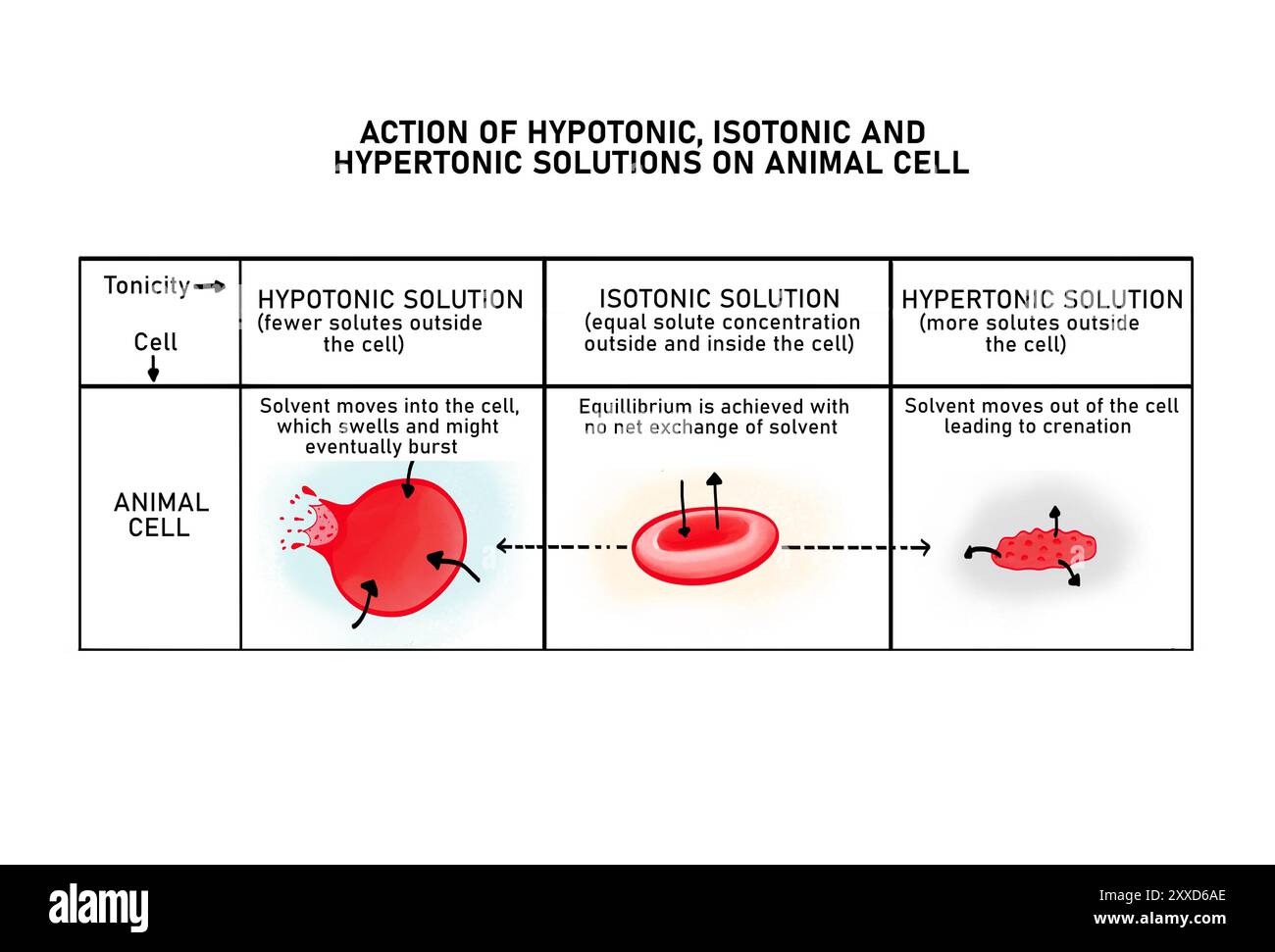
The Brutal Physics of a Red Blood Cell Placed in Pure Water
To understand why a red blood cell placed in pure water fails so spectacularly, you have to look at the cell membrane. Imagine a balloon that is "leaky" but only for water. This is a semi-permeable membrane. Inside the red blood cell, you have a concentrated soup of hemoglobin, salts, and proteins. Outside, in the pure water, you have... nothing. Just $H_2O$.
Nature hates a gradient.
Osmosis is the movement of water from an area of low solute concentration (the pure water) to an area of high solute concentration (the inside of the cell). The water isn't trying to be mean. It's just trying to reach equilibrium. It rushes through the membrane via tiny channels called aquaporins.
Because the red blood cell has no way to pump that water out fast enough, it swells. It turns from its usual flexible, biconcave disc shape—sort of like a tiny doughnut without a hole—into a rigid sphere. Eventually, the internal pressure exceeds the strength of the lipid bilayer.
Pop. The cell membrane tears. The hemoglobin spills out into the surrounding liquid. What’s left behind is an empty, translucent shell called a "ghost cell."
Why Tonacity Actually Matters for Your Health
We use the word hypotonic to describe pure water in this scenario. It literally means "under tension." In a hypotonic environment, the external fluid has a lower osmotic pressure than the fluid inside the cell.
Compare this to an isotonic environment. This is the sweet spot. In your body, the "saltiness" of your blood is roughly equivalent to a 0.9% sodium chloride solution. At this level, water moves in and out of the cell at the same rate. The cell stays happy and functional.
Then there’s the hypertonic scenario. If you’ve ever soaked in a salty bathtub for too long and your skin got pruney, you’ve seen a mild version of this. If you put a red blood cell in very salty water, the water rushes out of the cell to dilute the salt outside. The cell shrivels up like a raisin. Scientists call this crenation.
Real-World Medical Consequences
You might think, "When would I ever have pure water in my veins?" It happens more often than you'd think in clinical errors or specific drowning scenarios.
In cases of "freshwater drowning," a person breathes a large amount of lake or river water into their lungs. This water crosses the thin membranes of the lungs and enters the bloodstream. Suddenly, the blood becomes hypotonic. Thousands, or even millions, of red blood cells begin to burst simultaneously.
This is a double-edged sword of a medical emergency.
- You lose the ability to carry oxygen because the red blood cells are gone.
- The bursting cells release a massive amount of potassium into the bloodstream (hyperkalemia).
High potassium levels are incredibly dangerous for the heart. It can cause the heart muscle to stop beating entirely. This is one of the primary reasons why freshwater drowning victims often face heart failure even after they've been pulled from the water and started breathing again.
The Anatomy of a Red Blood Cell (And Why It Can't Cope)
Red blood cells, or erythrocytes, are specialized. They are basically sacks of hemoglobin. Unlike almost every other cell in your body, they don't have a nucleus. They don't have mitochondria. They traded all their internal machinery just to have more room for oxygen.
This makes them efficient, but also fragile.
👉 See also: Is Drinking Salt Water Bad for You? The Truth About This Dangerous Health Trend
A plant cell, for example, reacts very differently when placed in pure water. Plant cells have a rigid cell wall made of cellulose. When water rushes into a plant cell, it creates turgor pressure. The cell swells, but the wall holds it together. This is actually why plants stand up straight. When you don't water your flowers, they lose that pressure and wilt.
Human cells don't have that "external skeleton." We rely on the chemistry of our fluids to maintain our shape.
What Research Tells Us
Studies in the Journal of General Physiology have mapped the exact speed of this collapse. The membrane of a red blood cell is remarkably elastic, but it has a "critical hemolytic volume." Once the cell expands to about 140% of its original size, the structural proteins (like spectrin and actin) that support the membrane snap.
Honestly, it's a miracle our bodies maintain this balance as well as they do. Your kidneys are the unsung heroes here. They constantly filter your blood, dumping excess water or retaining salts to ensure that a red blood cell placed in pure water is a laboratory experiment, not a daily reality for your own anatomy.
Surprising Misconceptions About Hydration
There is a weird trend in some "extreme" wellness circles where people drink distilled water exclusively. The idea is that because it's "pure," it's better for you.
While drinking distilled water won't make your blood cells explode—because your stomach and intestines regulate the absorption and mix it with digestive juices—it can still mess with your electrolyte balance over time. Your body needs those minerals. If you only drink pure $H_2O$ with zero mineral content, your body has to pull minerals from your bones and tissues to keep your blood isotonic.
It’s basically the same principle of osmosis, just played out on a slower, more systemic scale.
How to Apply This Knowledge
Understanding the fate of a red blood cell placed in pure water isn't just for passing a biology quiz. It has practical applications for how you treat your body.
- Hydrate with Electrolytes: If you are sweating heavily during a workout, you aren't just losing water; you're losing salt. Drinking massive amounts of plain water can lead to hyponatremia, a condition where your blood becomes too "thin" (hypotonic). This leads to brain swelling and, in extreme cases, death. This is why Gatorade and other sports drinks exist.
- Medical Awareness: If you are ever caring for someone who is severely dehydrated, don't just give them plain water if they can't eat. They need a balance. Saltine crackers and water are a classic combo for a reason.
- Lab Safety: If you're a student or hobbyist working with blood samples, always use a buffered saline solution (like PBS) for dilutions. Using tap water will ruin your sample instantly, leaving you with nothing but cell debris under the microscope.
The take-away is pretty simple: balance is everything. Your cells are tiny, high-pressure machines designed for a very specific environment. Move them too far out of that environment, and the physics of the universe will literally tear them apart.
Next Steps for Better Health Maintenance
Check your hydration habits. If you find yourself drinking more than 4 liters of water a day without consistent salt intake, you might be stressing your kidneys' ability to maintain an isotonic state. Switch to mineral water or add an electrolyte powder once a day to ensure your red blood cell integrity remains optimal. Monitor for symptoms like "brain fog" or muscle cramps, which are often the first signs that your internal salt-to-water ratio is drifting into the hypotonic danger zone.